The Biological Resource Center of Institut Pasteur. The CRBIP is a transversal biobank infrastructure that includes microbial and human specimen collections of Institut Pasteur. The CRBIP receives, processes, maintains, characterizes and supplies biological resources globally, in compliance with health and environmental safety standards and under applicable laws and regulations.


The CRBIP has a unique position at national and international level, based on the combination of:
The CRBIP biological resources are integrated, processed, characterized, preserved and distributed in compliance with health and environmental biosafety and biosecurity standards and under applicable laws and regulations. Explore our large and diverse curated collections of microbial strains and human biological materials.
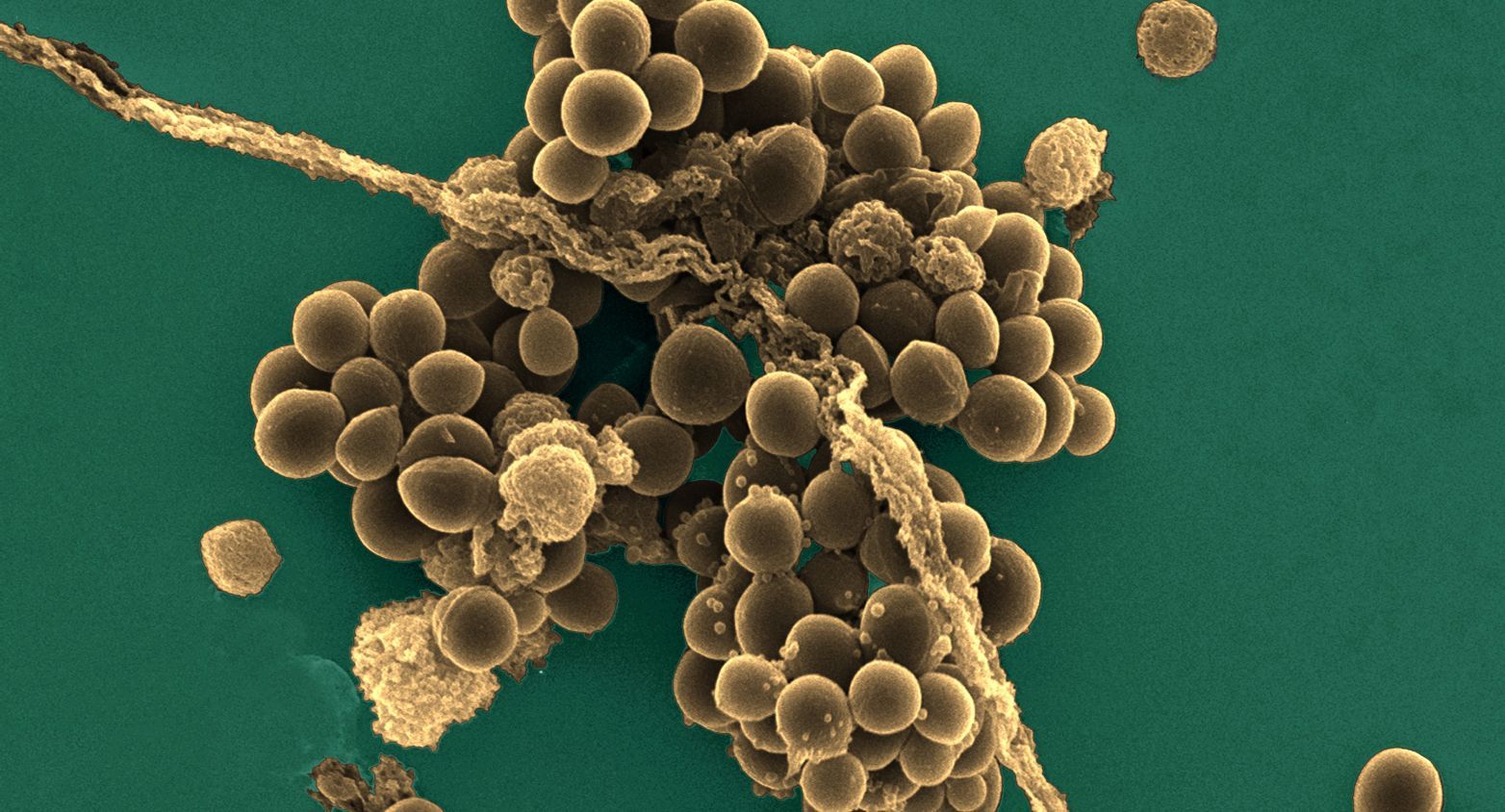
More than 15000 quality controled strains of bacteria.
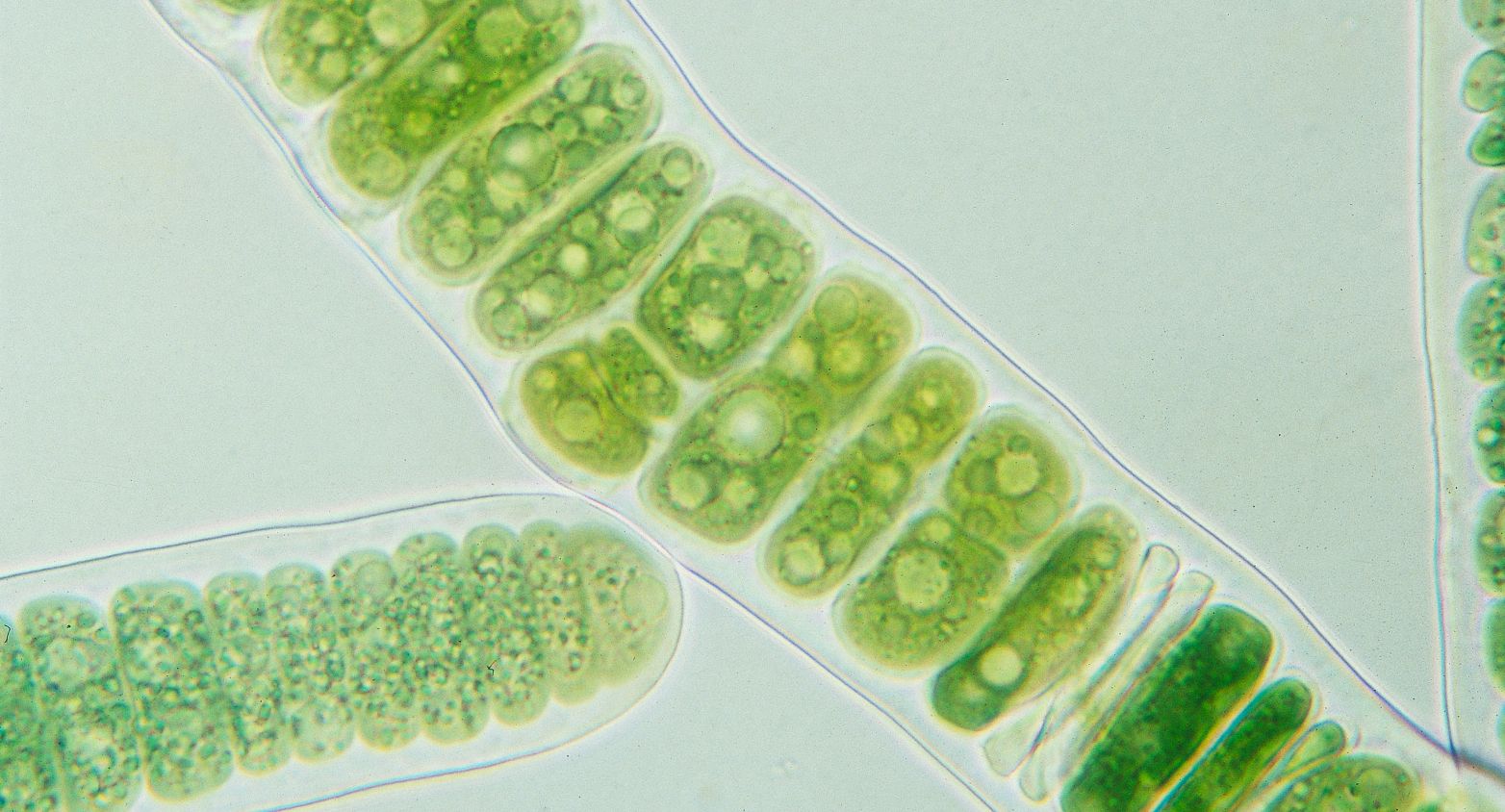
More than 700 axenic strains of cyanobacteria.
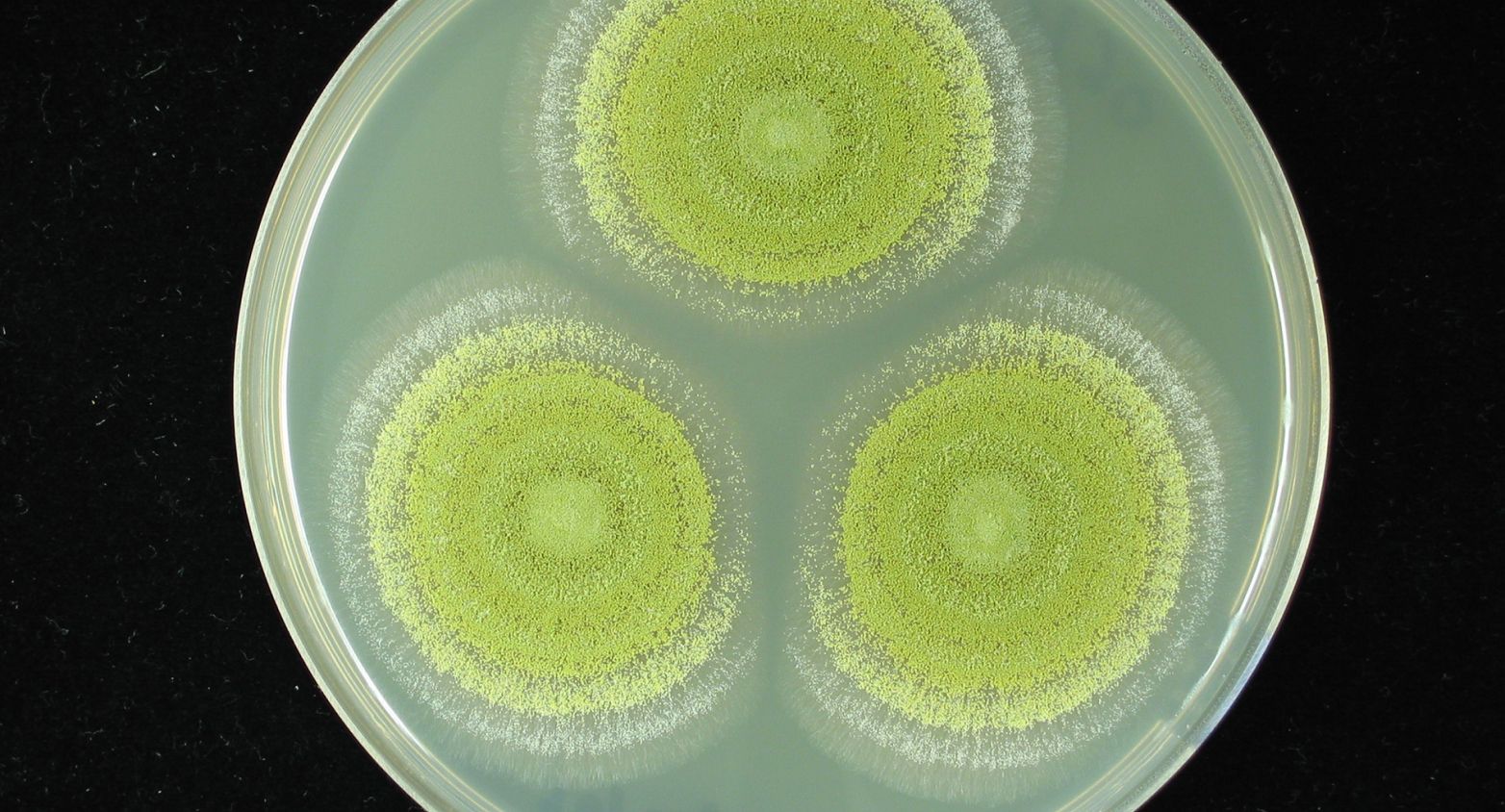
100 quality controled strains of fungi and more coming.
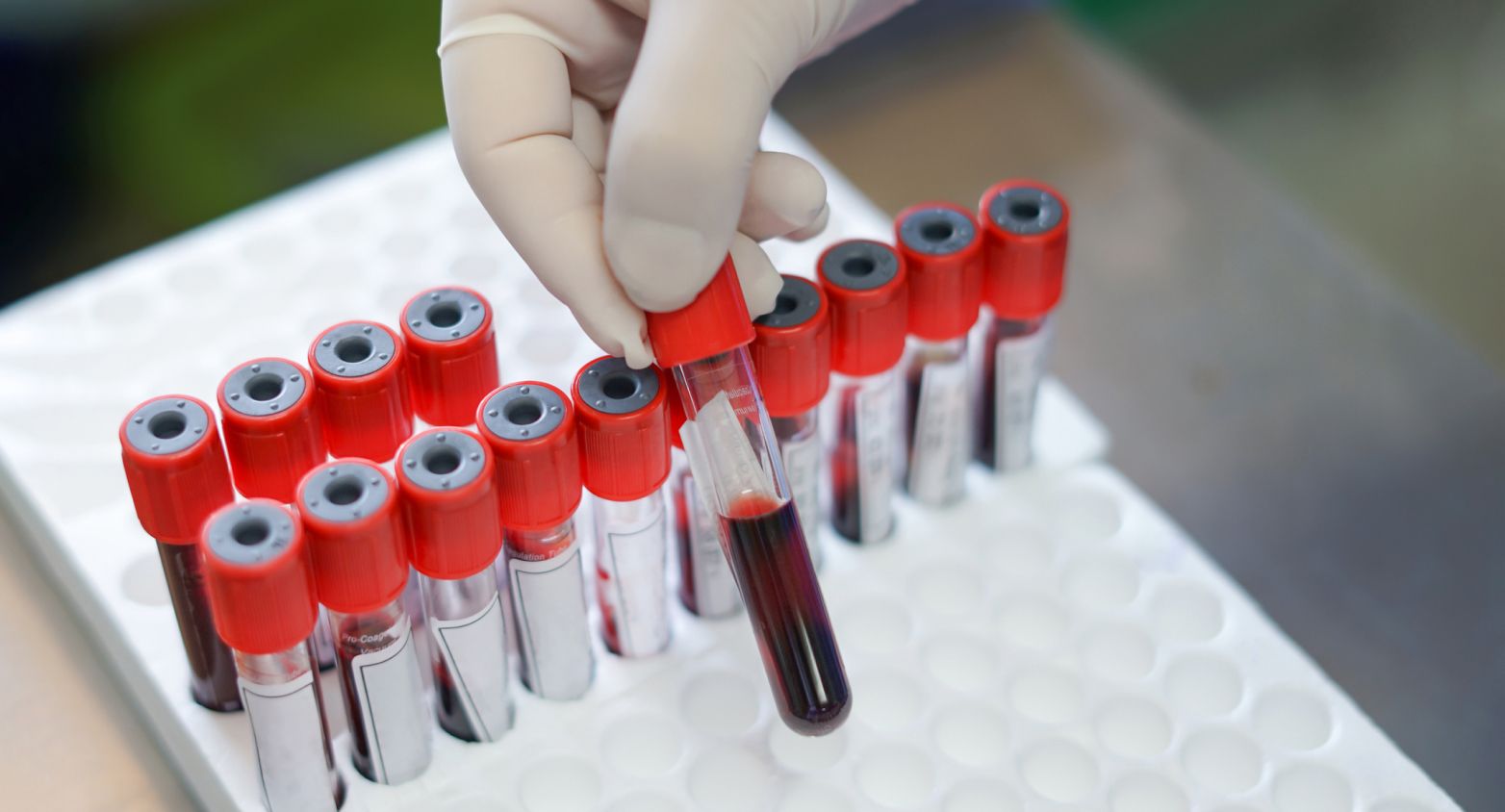
More than 200000 samples in stock.
CHIP catalogue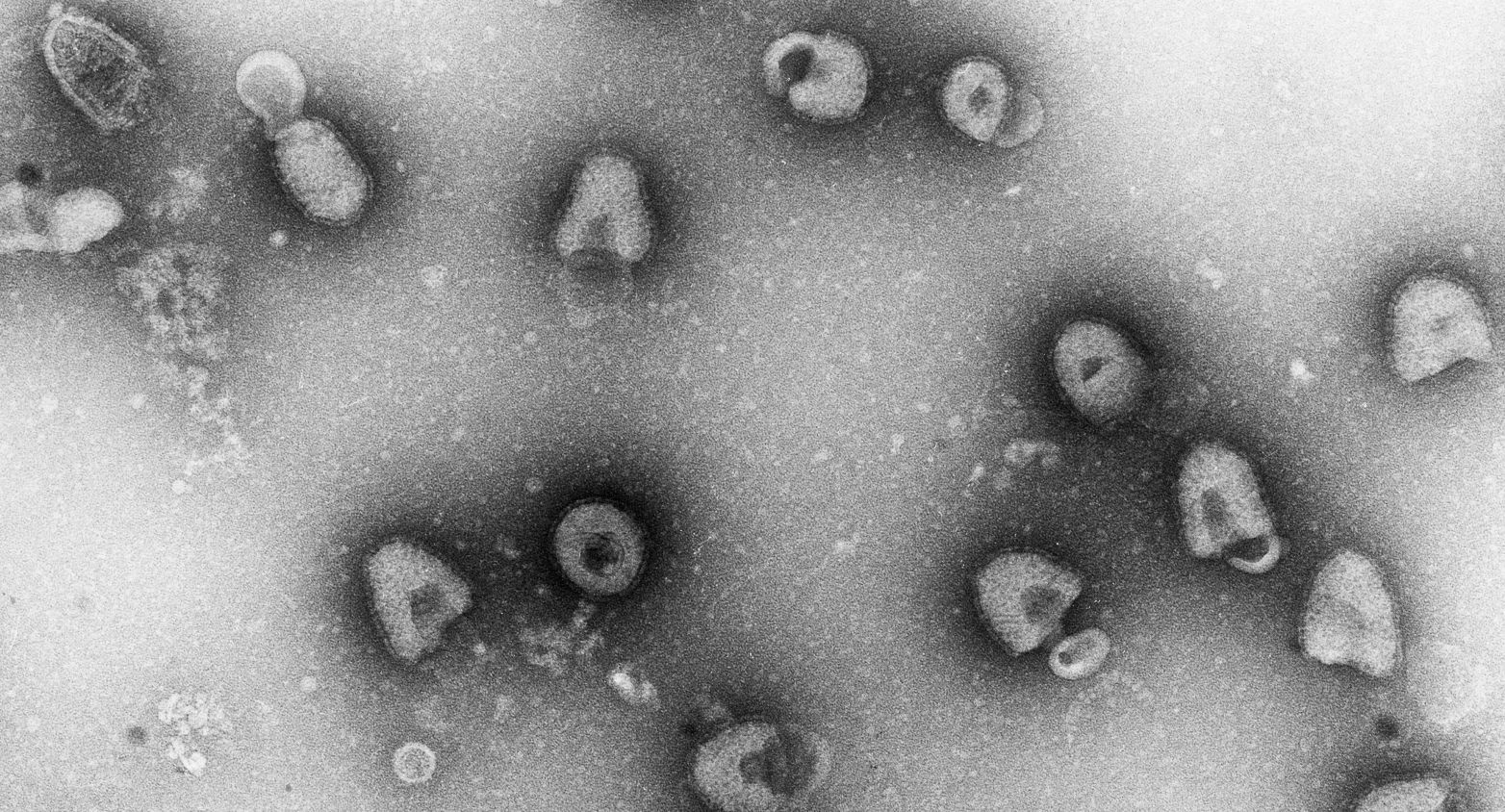
More than 200 viruses in EVAg.
EVAg catalogue (European Virus Archive - Global)
Bacteriophages and microbiome collections are planned.







The PARADIGM kick-off meeting took place on the 6th October 2024 at the Saint-Antoine Hospital in Paris (AP-HP).
Within the PARADIGM project, the Collection of Institut Pasteur (CIP) will be responsible for compiling and managing this national biobank for bacteriophages and hosting bacterial strains. The project management office ( CRBIP-PMO) will be involved in CRBIP internal coordination. The Genome Informatics and Phylogenetics Expertise Group (GIPhy) will be responsible for bioinformatics quality control of the phages and their host strains.
The PARADIGM project is supported by the French government under the France 2030 program managed by the National Research Agency (ANR), reference ANR-24-PEBI-0005. The project was selected by the ANR via its Priority Research Programs and Equipment initiative on Biotherapies – PEPR Biothérapies.
Discover the project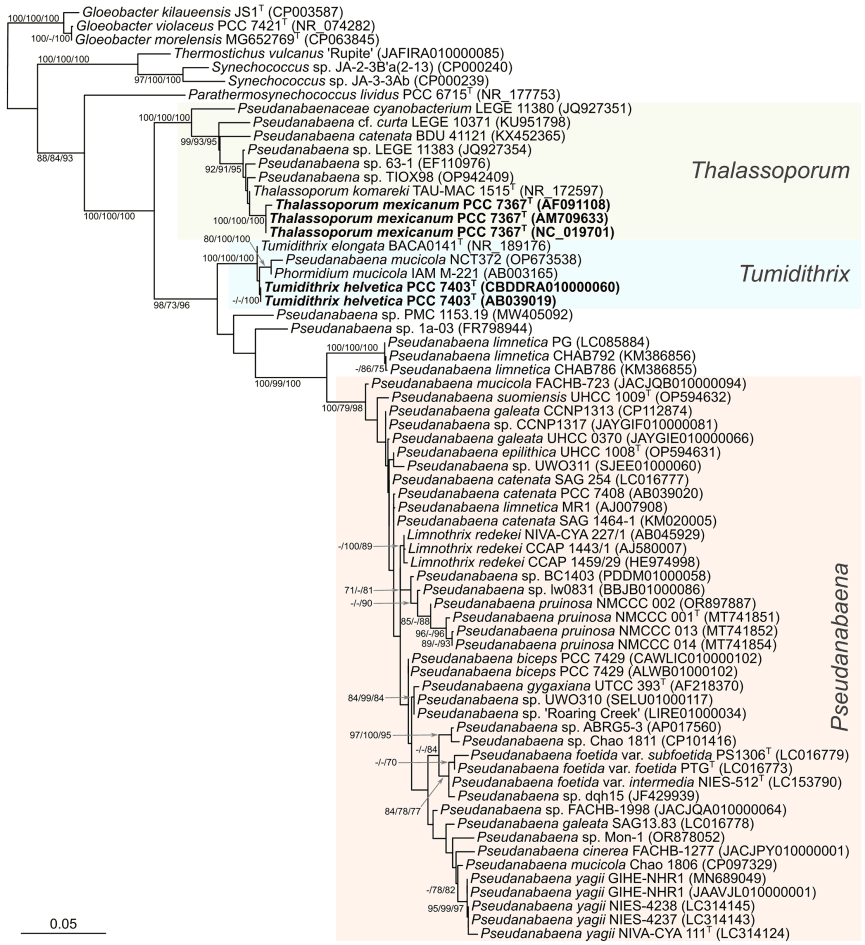
The taxonomic characterization of two novel Cyanobacteriota species has been recently published in the International Journal of Systematic and Evolutionary Microbiology (IJSEM).
This polyphasic evaluation, conducted by members from the Collection of Cyanobacteria (PCC) and the group 'Genome Informatics & Phylogenetics' (GIPhy), has successfully shown that the strains Thalassoporum mexicanum PCC 7367(T) and Tumidithrix helvetica PCC 7403(T) represent novel species of the pseudanabaenalean families Thalassoporaceae and Pseudanabaenaceae, respectively.
Discover the article
As part of valorization actions at CRBIP, the Genome Informatics and Phylogenetics (GIPhy) expertise group implemented large-scale bioinformatics strategies to reanalyze bacterial strains from CIP that have been sequenced since 2016. As a result, 463 of these new genome assemblies (mainly type strains) were publicly distributed to the scientific community.
Find the genome assemblies hereThe concept of “Target Specimen Profiles” (TSPs) for biobanks collecting specimen panels for diagnostic applications has been published.
A target specimen profile (TSP) corresponds to the required characteristics of the specimen panels needed to demonstrate that a diagnostic kit meets the target product profile (TPP). TSPs can guide biobanks in the prospective collection of sample panels to support the development and validation of diagnostics.
Ten examples of TSPs, specific for infectious diseases, are provided in the Supplementary Materials.
Discover the article
With a poster presentation on our new microbial Biobank Information Management System (MBIOLIMS), one oral presentation on the upcoming phage bank, and an oral presentation on our newly developed “culling tool” for samples of human origin, a CRBIP delegation has participated in the ISBER annual meeting taking place at Montral, Canada, on May 12-16.

The CRBIP is pleased to announce that the reception of isolates of carbapenem-resistant Acinetobacter baumannii (CRAb) has started, as part of the ECDC Europe-wide surveys for the surveillance of multidrug-resistant (MDR) bacteria.
These isolates are collected from acute care hospitals in European countries for the ECDC CRAb survey 2024–2025, an activity of the European Antimicrobial Genes Surveillance Network (EURGen-Net).
The CRBIP will performs tests of purity, viability and identity of the received isolates; maintains long-term, safe seed stocks of the isolates; and performs antimicrobial susceptibility testing (AST).

A new biobanking MOOC is launched by CRBIP and the Training Department of Institut Pasteur. For more information and to register, click on the button below.
Biobanking - Course - FUN MOOC
A warm welcome to Dr Meriem Paris who joined the CRBIP as the new Head of the CIP. Dr Paris brings her experience in microbiology across different types of microorganisms, biotechnology, management of microbial collections, biosafety/biosecurity and regulatory compliance.

Warm congratulations to Dr Mariana Ferrari who was appointed as the new Head of the CRBIP Project Management Office. Dr Ferrari is a microbiologist and cell biologist by training and has long experience in building and coordinating successful multi-partner collaborations in biobank infrastructures.

The CIP and CRBIP PMO Units of CRBIP work together in the operations of the central biobank for the bacterial isolates that are collected in the context of the ECDC genomic-based surveys.
For the first survey on carbapenem-resistant Acinetobacter baumannii (CRAb) in Europe, click on the button below.
Discover the protocol
The CHIP Unit of CRBIP is excited to be a partner in the new IHI project COMFORT.
This project focuses on patient-centric blood microsampling techniques, which collect less than half a millilitre of blood. For more information, click on the button below.
Follow this link
The CRBIP is officially member of the European Liquid Biopsy Society.
Extracellular vesicles (EVs) from human or microbial cells are part of liquid biopsies and represent important biomarkers in many human diseases.
ELBS website




We offer wet lab services for different types of biological resources, both microbial and human-origin. We also offer bioinformatics services for microbial resources.
The CRBIP accepts deposits of strains, and offers storage of your valuable or irreplaceable biological materials.
Processing of microbial or human specimens, preparation of molecular or cellular derivatives.
Authentication, quantification, phenotypic characterization, genomic taxonomy services
Strict Quality Assurance is a central part of our strategy. Our Quality Management System covers all biobanking processes and aims at fitness-for-purpose of the biological resources we are making available.


for any question to which you do not find the answer in the FAQ, don't hesitate to contact us, crbip-pmo@pasteur.fr
Access to our FAQ



